
In dentistry, as in any medical discipline, the risk of cross infections is always right around the corner and it is necessary to implement all the disinfection and sterilisation strategies required to minimise the possibility of infection for practice staff and for the patients themselves.
A large number of people come in and out of a dental practice each day, each one with their own patient history, which should really be taken into consideration to draft a precise and appropriate treatment plan for each specific situation. Collecting patient history is therefore essential to preventing infection, but it is also for a number of reasons highly recommended to treat each patient with the care and precaution we would use for a patient with an acute, established infection.
Formation role in dentistry
In every dentist’s everyday clinical practice, in addition to the technical and operating expertise, the in-depth knowledge of the most basic hygiene measures and sterilisation/disinfection processes also plays a key role, especially in prevalently surgical practices. In all facilities, it is vital that the practice staff have been suitably trained and therefore able to apply hygienic practices impeccably, thereby contributing towards keeping the workplace safe for both the patients and the health staff who work there.
To illustrate the risks in such places, mention must be made of the Istituto Superiore di Sanità (Italian Higher Institute of Health), which in 2000 recognised dentistry activities as among those at high risk relating to the possibility of transmitting or contracting hepatitis.
So when a clinical worker approaches the purchase of specific equipment and products, (s)he should think outside the box and look beyond the propaganda of each manufacturer and most of all form his/her own opinion on disinfection and sterilisation and avoid delegating the decision to staff members who are not sufficiently qualified to make such a crucial decision for the safety of all those who move in and out of the practice. The correct and appropriate use of the tools at our disposal guarantees greater protection of the health of patients and operators themselves, while at the same time limiting undesired toxic effects and frequently also operating costs.
Sterilisation process
Preparatory phases
All medical devices that penetrate tissues or the vascular system and which in any case come into contact with blood, secretions, the skin and mucous membranes must undergo a decontamination and sterilisation process, and must be stored in a clean, well-organised dedicated place.
The process to follow before performing the actual sterilisation consists in several stages:
- Decontamination
- Washing
- Rinsing
- Drying
- Checking
- Maintenance
- Packaging
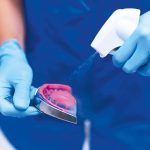
Decontamination should be carried out using liquids compliant with the reference technical standards, with virucidal, bactericidal and fungicidal activities; this first step drastically reduces the risk of transmitting HIV, HBV, HCV and other pathologies.
Before washing, instruments consisting of multiple parts must be disassembled so that the sterilisation process is feasible in all parts.
Washing can be manual, but it poses a higher risk for practice staff who are therefore more susceptible to potential damage and infection, or through the use of instrument-washing or heat-disinfection machines which reach temperatures close to 100° and can also use dedicated detergents and disinfectants.
After washing, the material must be first rinsed under a running water shower and then showered with demineralised water, to remove all detergent residue. After rinsing, the material is dried thoroughly with compressed air guns or paper or fluff-free canvas towels. The last step before autoclaving the instruments is a careful check of all instruments, of all their parts, and their maintenance through the application of specific products.
Bagging and sterilisation
When these processes have been completed, each instrument must be bagged and sterilised; during this phase, the traceability of the process is important, so every single bag needs to be identified uniquely with a code and the date of the day of the sterilisation process.
After 30 days, the sterilisation process can be deemed no longer reliable and should be repeated before the instruments are used again. In dentistry, moist heat autoclaves are used for this purpose, as they are the most cost-effective, simple way which is what the majority of practices opt for. These instruments are designed to sterilise the materials placed inside them in relatively short cycles (approximately 20 minutes).
Do you want more information on Zhermack Dental products and solutions?
Contact us


 Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.
Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.


