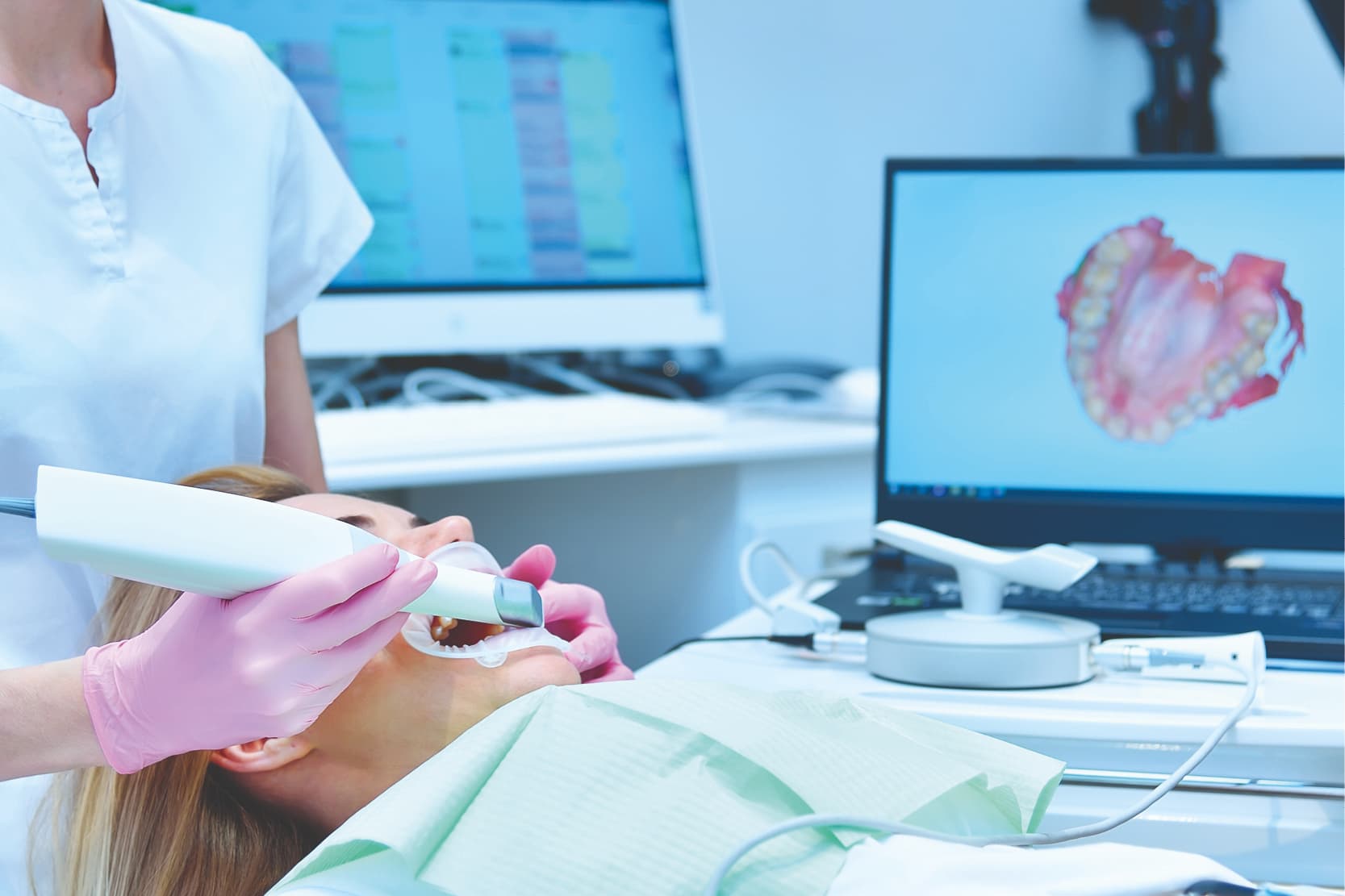
Today, professionals operating in the dental sector work with a variety of digital technologies able to streamline healthcare procedures by making them more fluid and manageable (inbuccal X-rays, intraoral scanners, electronic clinical records, etc…).
Just like conventional instruments, technological tools must also be managed in accordance with the procedures set out in guidelines on the prevention of infection, as well as the instructions provided by the manufacturer of the device.
Being familiar with these instructions is a fundamental part of effective infection control, because if they are not carried out in accordance with the manufacturer’s guidance, the devices could pose a risk of cross-transmission.
An effective infection control policy for dental technology will ensure the safety of the patient and the practitioner during treatment.
Impermeable barriers and ready-to-use disinfectants: the alternatives to heat sterilisation
Each device comes with its own instructions regarding sterilisation or disinfection and oral health professionals should keep in step with the updates to infection control guidelines.
Although heat sterilisation should be used for semicritical articles when possible, many devices have a combination of critical parts and non-critical parts that cannot withstand heat sterilisation.
Disinfection of computer mice, keyboards and other electronic devices
For example, the computer mouse and keyboard used in operating theatres to manage electronic clinical records can be a source of cross-infection,[1] because these surfaces can come into contact with most of the microorganisms present during appointments with patients and retain them, increasing the risk of contamination.
These surfaces should be protected with barriers before and after use and they must be disinfected after one patient and before the other[2].
Impermeable plastic barriers are an effective way to prevent the cross-contamination of devices.The guidelines issued by the CDC (Centers for Disease Control and Prevention) in 2003 emphasise that barriers are effective for difficult-to-clean articles, like electronic devices[3].
For other instrumentation, such as:
- intraoral scanner handpieces;
- infrared lamps;
- devices for screening of cancer of the mouth;
- curing lamps;
- portable X-ray units;
- intraoral video cameras;
it is necessary to comply with the disinfection and sterilisation protocols provided by the manufacturer, since this instrumentation consists of parts that can be damaged by heat (or steam), which could, therefore, have an adverse effect on the functioning of the device.
Zhermack’s Zeta Hygiene line provides a range of disinfectant and detergent products in ready-to-use form (Zeta 3 Soft and Zeta 3 Foam) and in wipe form (Zeta 3 Wipes TOTAL and Zeta 3 Wipes POP-UP), specifically designed for the cleaning and disinfection of the surfaces of even the most delicate medical devices.
Bibliography
[1] Reynolds KA, Watt PM, Boone SA, Gerba CP. Occurrence of bacteria and biochemical markers on public surfaces. Int J Environ Health Res. 2005;15:225–234
[2] Hartmann B, Benson M, Junger A, et al. Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J Clin Monit Comput. 2004;18:7–12.
[3] Kohn WG1, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings — 2003. MMWR Recomm Rep. 2003;52(RR-17):1–61.
Do you want more information on Zhermack Dental products and solutions?
Contact us


 Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.
Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.


