As things stand today, most rehabilitative processes in dentistry involve one or more phases distributed between the dental practice and the laboratory.
The dental technician is the lab professional who works in close collaboration with the dentist and produces various types of devices starting from a replica of the patient’s anatomy, as part of a surgical or dental prosthetic rehabilitation.
The more complex the rehabilitation, the more numerous and complex the phases of dental laboratory work.
The laboratory and its environments
Various kinds of dental device are produced in the laboratory. Labs therefore require many different items of equipment, most of which are quite complex. These have to be arranged inside the lab in a way that makes their use as ergonomic as possible and also ensures the safety of operators.
Generally speaking, small labs are divided into two main areas: the actual laboratory and a stone casting room. In addition to these two areas, many labs also contain a quiet, dust-free environment for the production of ceramic items and, with increasing frequency, a fourth area set aside for CAD/CAM purposes. (1)
To ensure an adequate level of safety, before starting to learn even the simplest process, new technicians need to be instructed in basic safety precautions concerning the equipment and materials they will be using. (1)
It is essential to carefully evaluate whatever hazards cannot be eliminated while sources of risk remain. All operatives must therefore be informed and made aware of the risk factors connected with laboratory activities and must strive to minimise risk through the responsible use of materials and equipment, and above all by wearing the PPE (personal protection equipment) that can best safeguard them in their work. (1)
The importance of information
The better informed a worker is, the more safely he will work, precisely because he is motivated to protect himself. (1)
In Italy, occupational safety is regulated by the “Law on occupational health and safety”, Decree-Law 81 of 9 April 2008 (most recently updated in April 2022). This Law replaced a previous Decree-Law, 626 of 1994. The law clearly emphasises the importance of prevention as the primary source of safety at work, and generic precautions are established in Article 15. (1)
Dental lab technicians are exposed to various injury risks (cuts, abrasions, burns, electric shock, etc.) and occupational disease risks (visual and auditory pathologies and diseases caused by fumes, dust, poor ergonomics, etc.).
One of the hazards that dental technicians face on a daily basis is biological in nature. In brief, material received from dental practices may be a source of contamination and potential infection.
What physical risks are found in dental laboratories?
According to a review published in 2018 (2), a large percentage (around 60%) of the objects received from dental practices are contaminated and not disinfected (3, 4). Communication between practices and labs concerning disinfection processes was also found to be deficient (5, 6). The prevention of cross-contamination as a way of reducing infection is therefore of the greatest importance to both dentist and technician.
In addition, it is more likely for cross-contamination to occur between a dental practice and a lab than between a dentist and patient or between one patient and another. (7) Despite the absence of direct contact with patients, it has been seen that dental technicians are more liable to hepatitis B (2.7%) than the public at large (0.76%). (8)
The oral cavity is not a sterile environment. The microbial population of the mouth normally includes both harmless micro-organisms and etiologic agents behind serious infective pathologies. (9)
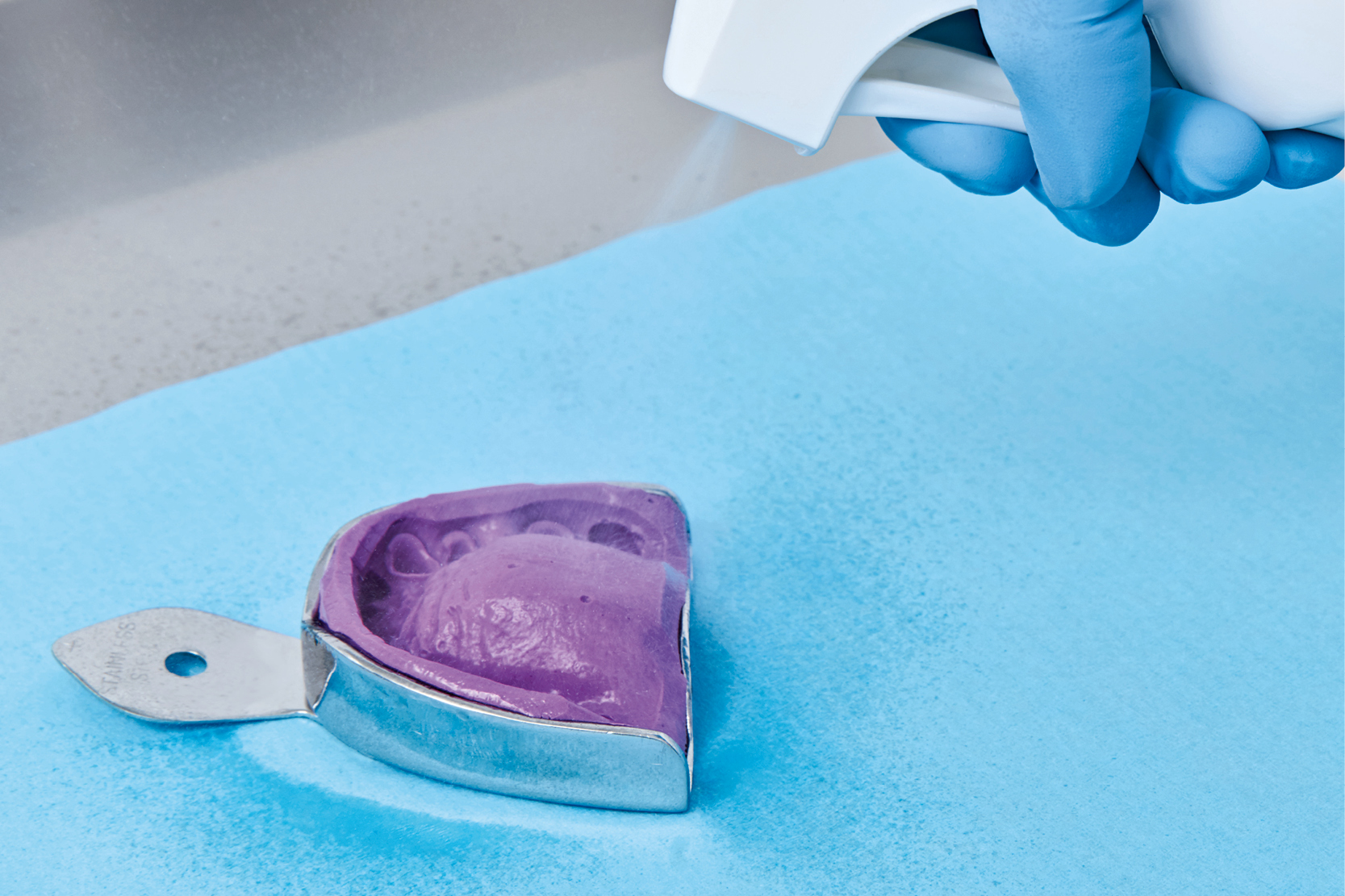
Communications between the dental practice and the lab are generally based on impressions of dental arches or existing prosthetic devices that need modification or repair. Impressions can be made from various materials but, to ensure an effective rehabilitation, such materials must be dimensionally stable. Given this fundamental prerequisite for prosthesis production, we must exclude physical sterilisation as a potential procedure for preventing infection. Physical sterilisation would inevitably cause deformation in the impression material and make it useless for its intended purpose. (9)
What safety precautions should a dental laboratory adopt?
Some form of disinfection is essential to reduce microbial load to a safe level. Some disinfectant products can cause dimensional alterations in impression materials, but provided the right products are used in the correct concentrations and for the prescribed times, no significant alteration occurs. (9)
A specific protocol must be followed whenever an impression arrives at the lab (9):
• Wear protective gloves to remove the impression from its packaging.
• Always unpack the impression in a specific area, used only to receive incoming work. This area must be
cleaned and disinfected regularly.
• Use a separate tray to transport each impression. Never carry more than one impression together.
• Disinfect the impression if this has not already been done by the dental practice.
It is worth remembering that the dentist is responsible for disinfecting impressions and for stating the method of disinfection on the prescription form accompanying the impression.
What safety legislation applies to dental laboratories?
On the international level, according to the guidelines published by the CDCs (Centers for Disease Control and Prevention), the correct PPEs (gloves, face masks and shields) must be worn to handle, clean, disinfect and rinse any object (impression, bite registration, prosthesis etc.) arriving at a laboratory from a dental practice. The disinfectant used must be of at least average strength. (10) Physical sterilisation should be used with materials that are not sensitive to heat.
It is therefore clear that precise and timely communication is essential between the dental practice and the lab, and that specific protocols must be adopted when handling material sent by practices to labs in order to reduce the risk of cross-contamination.
To disinfect impressions, Zhermack offers a range of high performance products like Zeta 7 Solution (for disinfection by immersion) and Zeta 7 Spray (for spray application). These products boast a broad spectrum of action, confirmed by testing to the latest European disinfection standards, and excellent compatibility with all kinds of impression material.
Bibliography
- https://online.scuola.zanichelli.it/ilcorpoumano2ed-files/il-corpo-umano-vol-2-odontotecnici/leggere-ebook/C_3_p69_Sicurezza_laboratorio_odonto.pdf
- Vázquez-Rodríguez, I., Estany-Gestal, A., Seoane-Romero, J., Mora, M. J., Varela-Centelles, P., & Santana-Mora, U. (2018). Quality of cross-infection control in dental laboratories. A critical systematic review. International Journal for Quality in Health Care, 30(7), 496-507.
- Sofou A, Larser T, Fiehn NE et al. Contamination level of alginate impressions arriving at a dental laboratory. Clin Oral Investig 2002;6: 161–5.
- Haralur SB, Al-Dowa OS, Gana NS, Al-Hytham A. Effect of alginate chemical disinfection on bacterial count over gypsum cast. J Adv Prosthodont 2012;4:84–8.
- Jagger DC, Hugget R, Harrison A. Cross-infection control in dental laboratories. Br Dent 1995;179:93–6.
- Akeredolu PA, Sofola OO, Jokomba O. Assessment of knowledge and practi ce of cross infection control among Nigerian dental technologists. Niger Postgrad Med J 2006;13:167–71.
- Hazelkorn HM, Bloom BE, Jovanovic BD. Infection control in the dental office. Has anything changed? J Am Dent Assoc 1996;127:786–90.
- Wilcox CW, Mayhew RB, Tiffany RL. Incidence of hepatitis B exposure among USAF dental laboratory technicians. Am J Dent 1990;3:236–8.
- https://www.eber.org/documenti/pubblicazioni/06_odontotecnici.pdf tratto da ddl 626 1994
- https://www.cdc.gov/oralhealth/infectioncontrol/pdf/recommendations-excerpt.pdf
Do you want more information on Zhermack Dental products and solutions?
Contact us



 Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.
Zhermack SpA has been one of the most important producers and international distributors of alginates, gypsums and silicone compounds for the dental sector for over 40 years. It has also developed solutions for the industrial and wellbeing sectors.
Zhermack SpA - Via Bovazecchino, 100 - 45021 Badia Polesine (RO), Italy.


